7th World Bronchiectasis and NTM Conference
The 7th World Bronchiectasis and NTM Conference provides education around epidemiology, pathogenesis, diagnosis, management and treatment of bronchiectasis and nontuberculous mycobacteria (NTM).






Chronic cough

Dyspnoea

Daily sputum production

Fatigue
Recurrent infections

Haemoptysis
Data from a study of 2,572 patients with bronchiectasis from 10 clinical centres across Europe and Israel showed that:13
Patients with 2 or more exacerbations per year at baseline had a 60% increased risk of 5-year all-cause mortality.
Patients with 3 or more exacerbations per year at baseline had an 86% increased risk of 5-year all-cause mortality.

Cough

Fatigue and/or malaise

Breathlessness and/or exercise intolerance

Haemoptysis

Sputum volume and/or consistency

Sputum purulence
Preventing exacerbations can help make a positive impact on patients both physically and mentally.7,13
It’s important that patients are educated about the consequences of exacerbations and the appropriate actions to take, including when to seek medical help and report them to their treating physician.
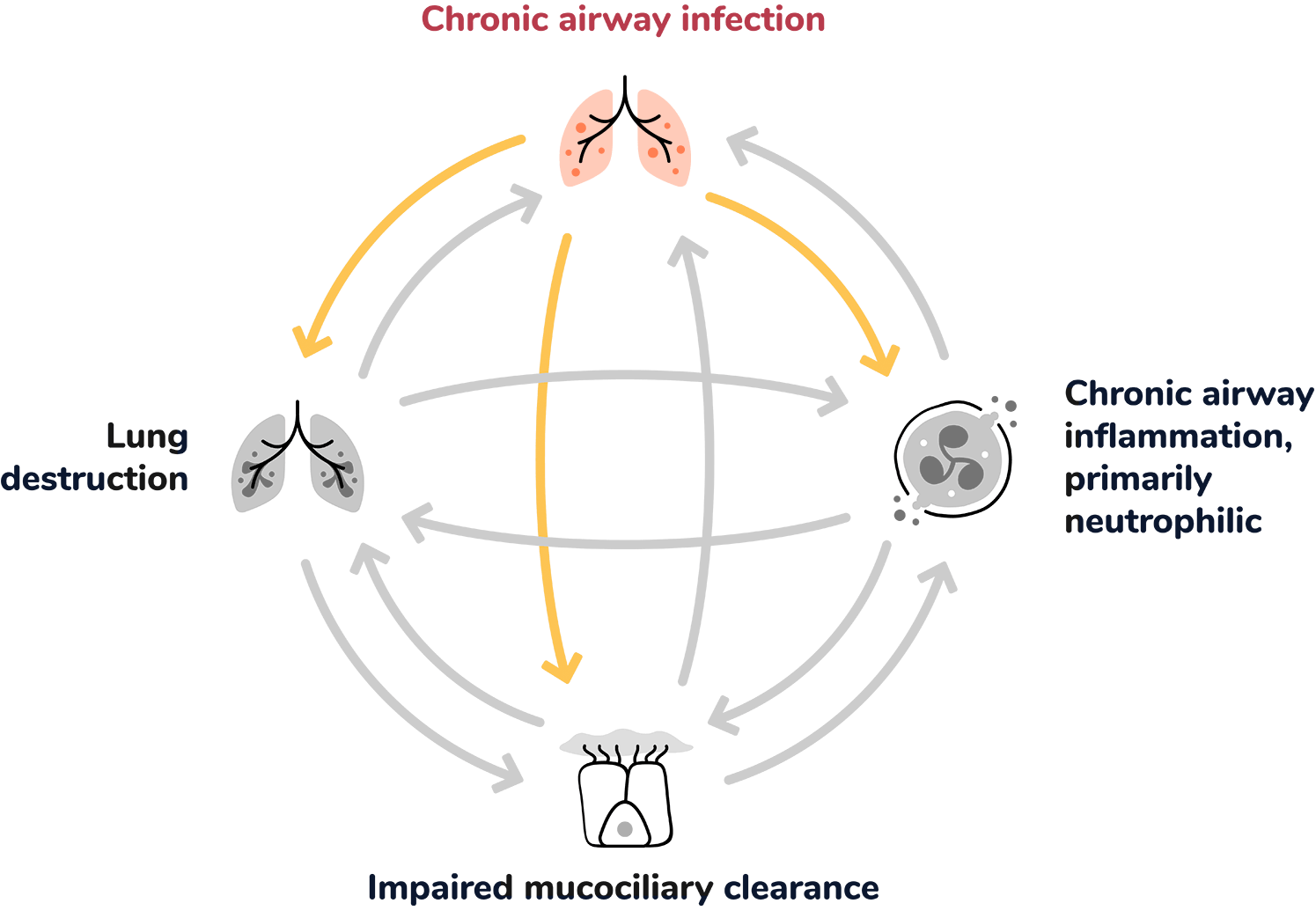
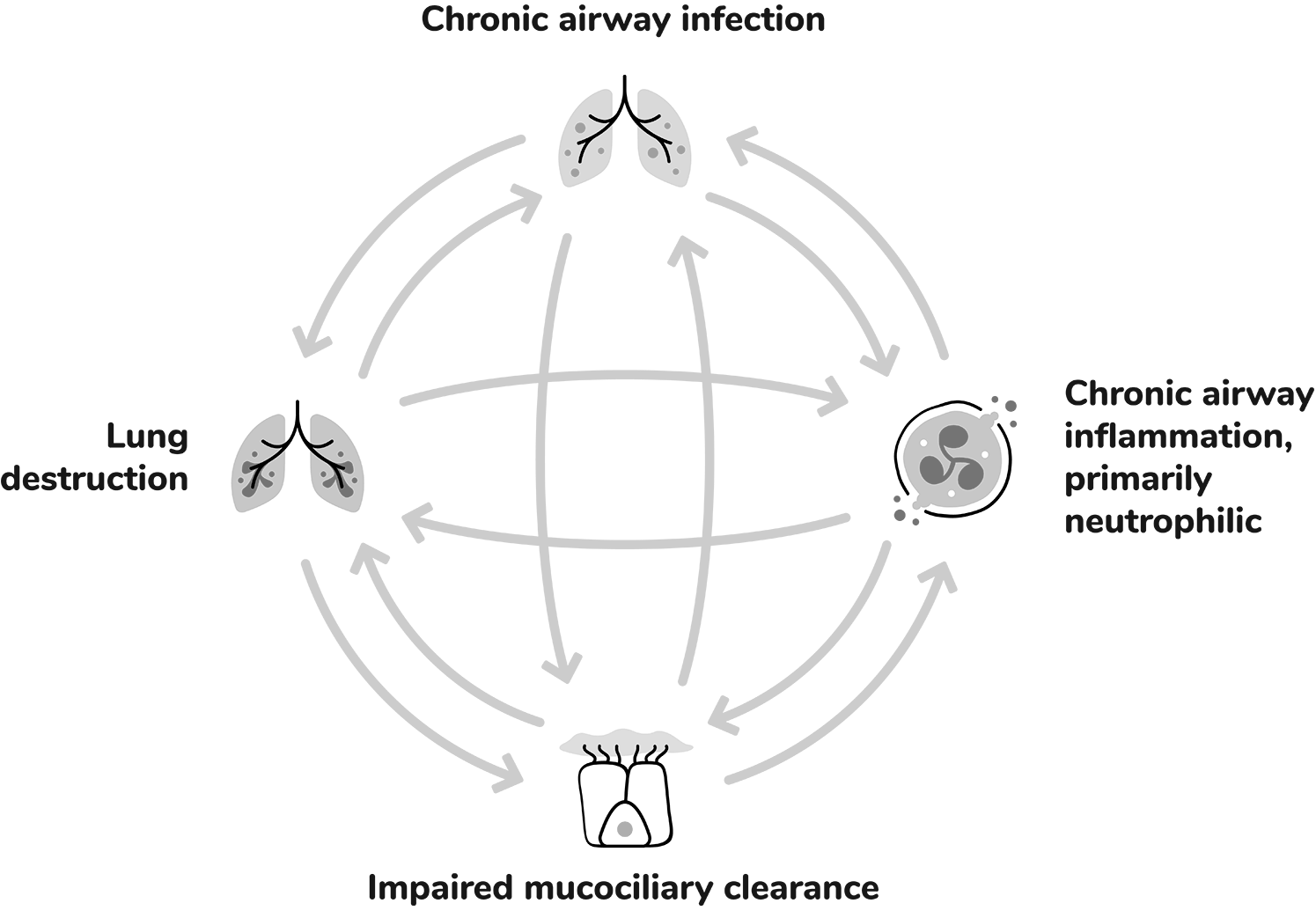
Within the self-perpetuating cycle of bronchiectasis, each driver can lead to the worsening of the others and contribute to progressive lung damage and exacerbations.1,5
Chronic airway infection contributes to the pathophysiology of bronchiectasis by inducing chronic airway inflammation, which can lead to progressive airway damage and injury.1
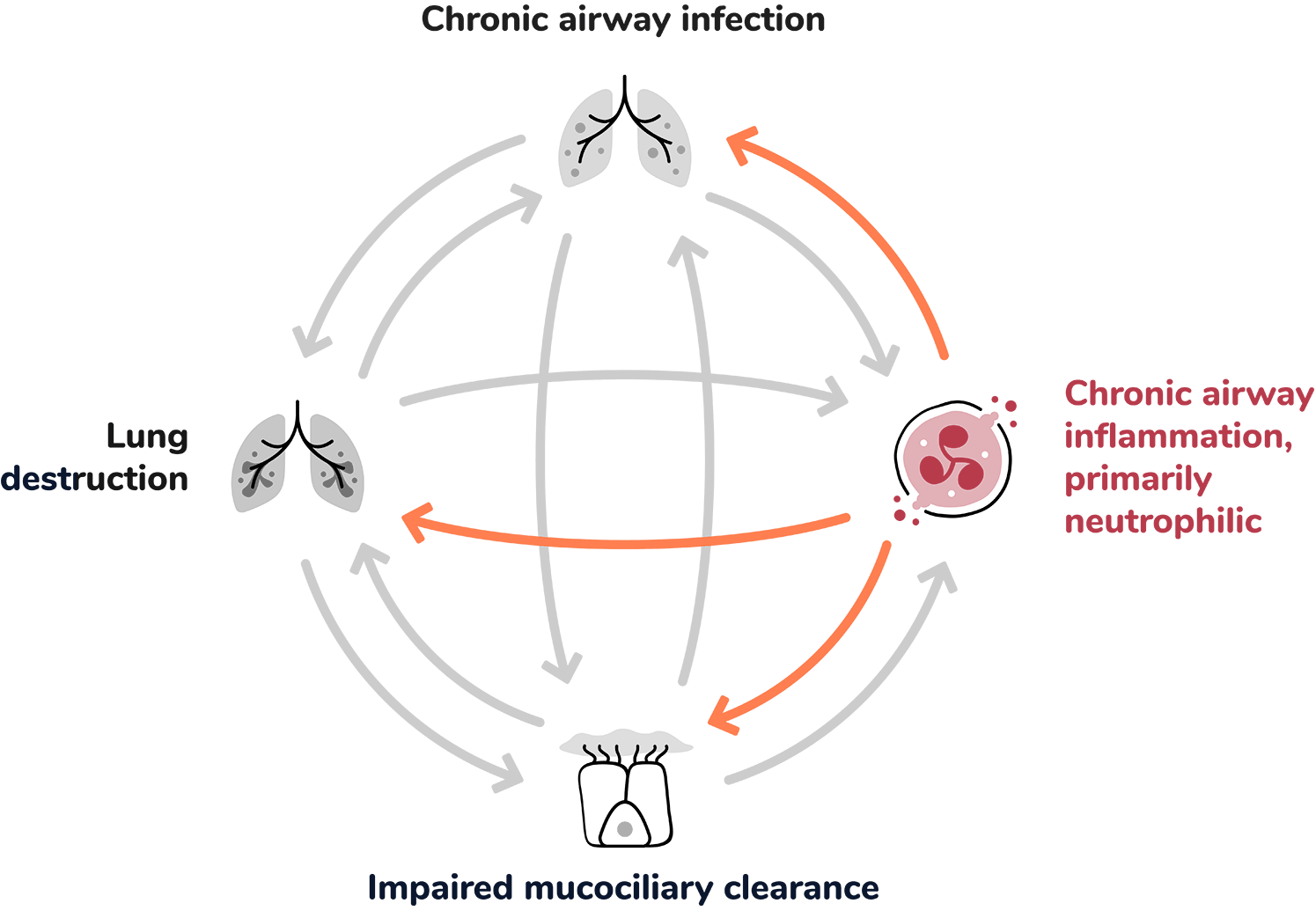
Extensive infiltration of the airways by inflammatory cells: 1,5,7
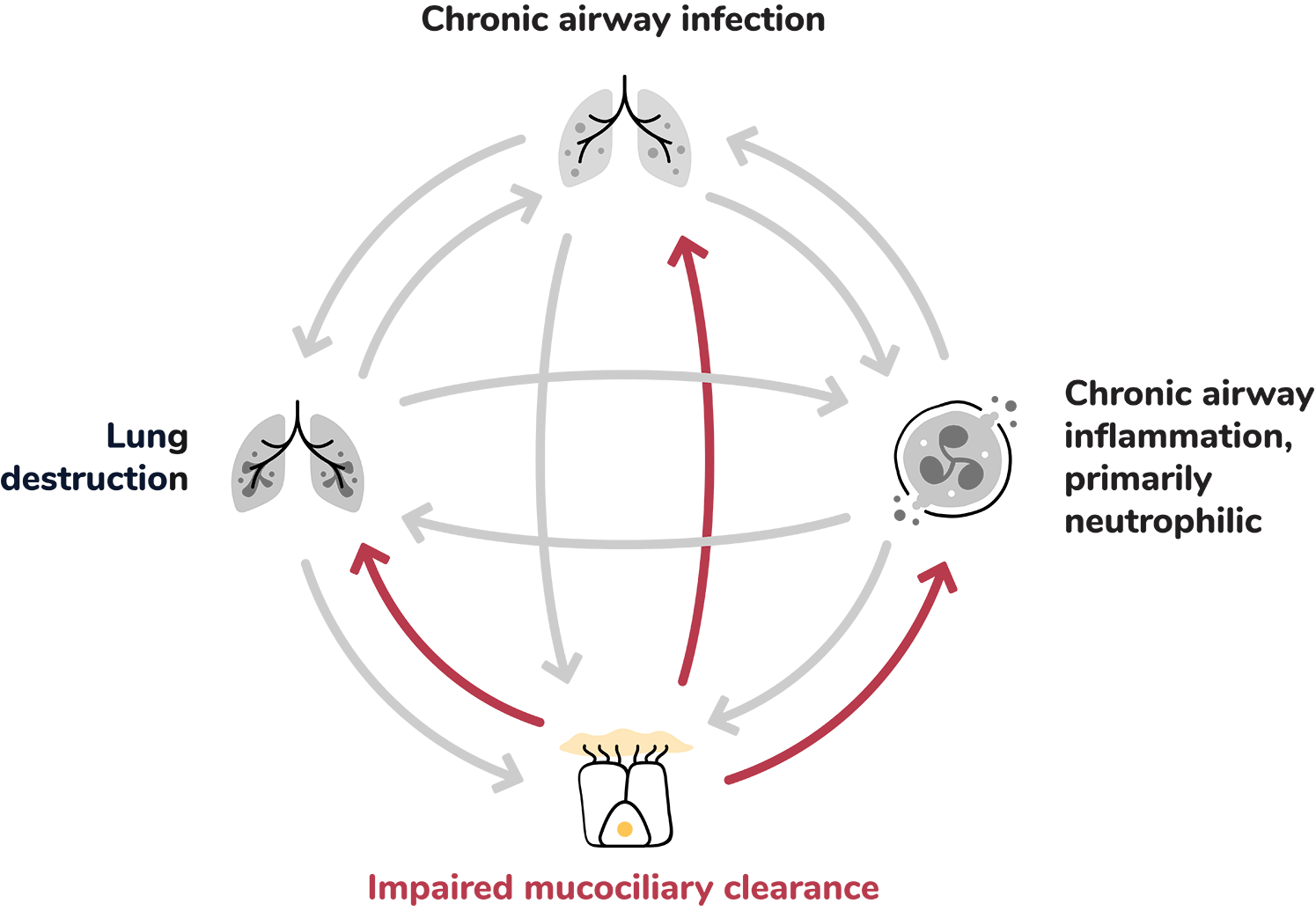
Dysfunctional mucociliary clearance can lead to sputum retention in airways, creating a harbour for infection and inflammation.1
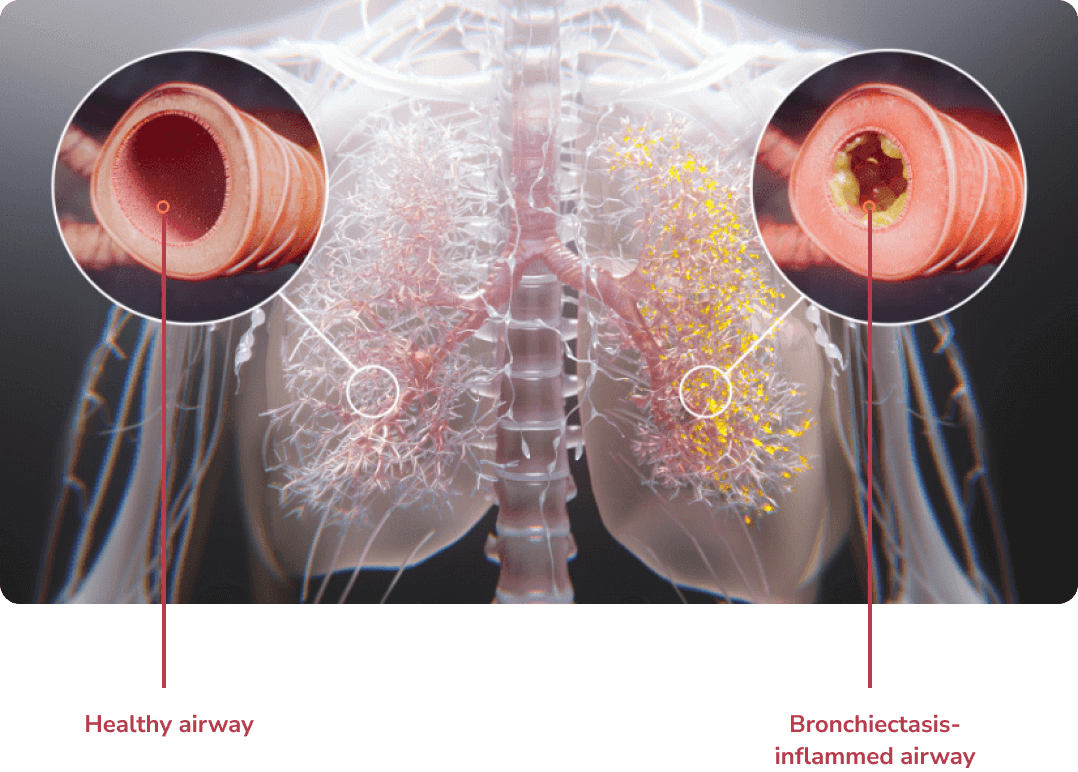
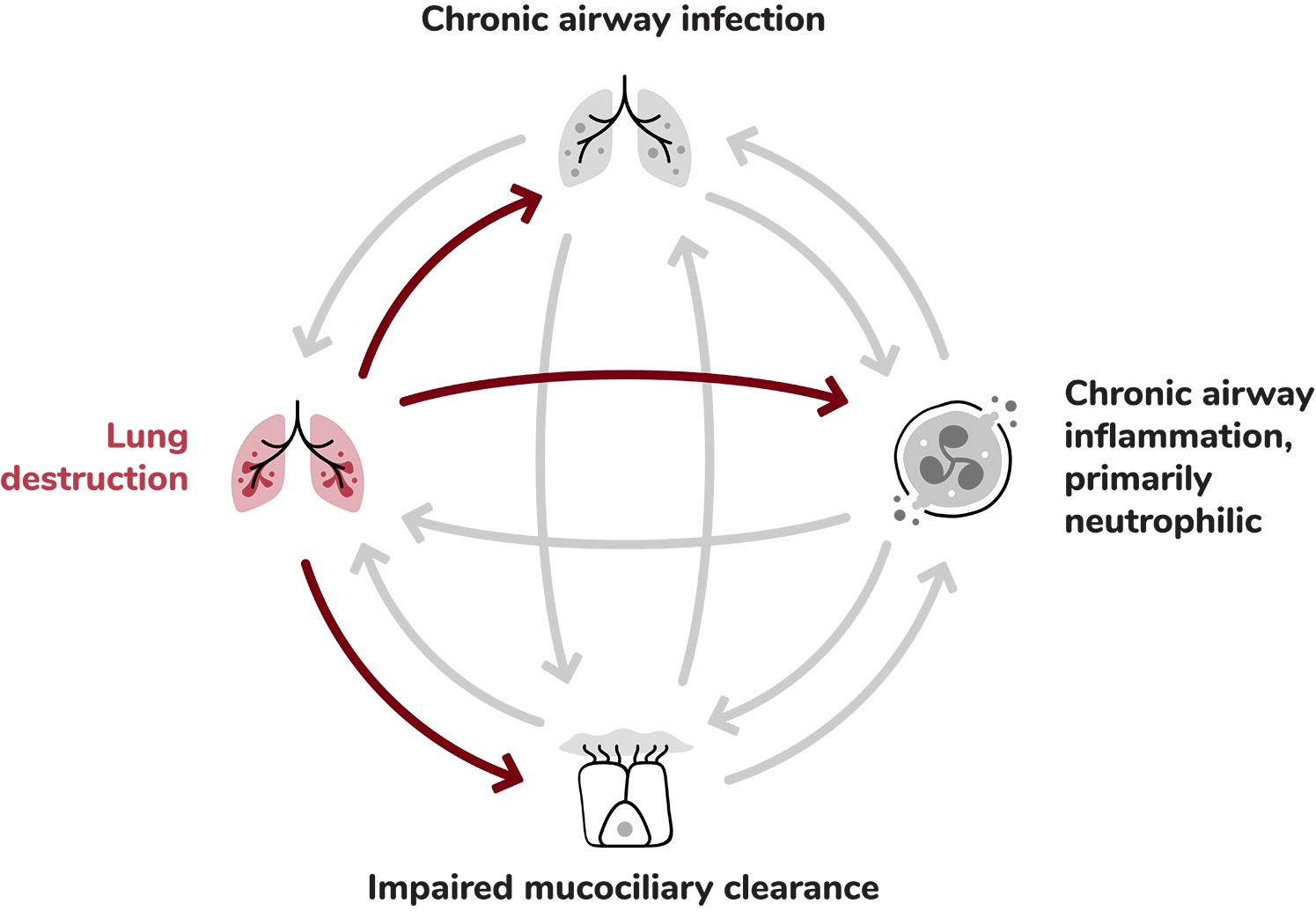
Structural lung damage involving bronchial wall destruction and dilation.1
Click to explore
Chronic airway infection contributes to the pathophysiology of bronchiectasis by inducing chronic airway inflammation, which can lead to progressive airway damage and injury.1
Extensive infiltration of the airways by inflammatory cells: 1,5,7
Dysfunctional mucociliary clearance can lead to sputum retention in airways, creating a harbour for infection and inflammation.1
Structural lung damage involving bronchial wall destruction and dilation.1
The overactivity of neutrophil elastase (NE) contributes to chronic inflammation, concomitant tissue damage and an increased risk of future exacerbations.2,3,6
Bronchiectasis is a chronic lung disease marked by permanent, abnormal dilation of the bronchi and chronic airway inflammation.1,2
While there are various aetiologies of bronchiectasis such as infection, COPD, asthma and GERD, in many patients the aetiology cannot be identified (idiopathic bronchiectasis).3,4
Common symptoms of bronchiectasis include a chronic cough with sputum production, dyspnoea, fatigue and haemoptysis.3,5
Many patients suffer from repeated exacerbations, generally defined by an increase in daily respiratory symptoms and potential change in therapy. Increased frequency of exacerbations can be associated with disease progression and reduced quality of life.6,7
A diagnosis of bronchiectasis requires both the presence of signs and symptoms such as chronic productive cough or history of exacerbations and radiological evidence often via a high-resolution computed tomography scan.5
Bronchiectasis pathophysiology has been described in literature as a ‘vicious cycle’ or ‘vicious vortex’, consisting of four interconnected components:8,9
Abnormal mucus production and mucociliary clearance, where thick mucus becomes trapped in the airways which renders the airway more susceptible to chronic infections.8
The inflammatory response is complex. It is primarily neutrophilic, but also involves a network of cytokines and other inflammatory cells, including macrophages, eosinophils and lymphocytes. Collectively these add to airway damage and lung destruction.8
Within this self-perpetuating process, each component can contribute to the worsening of the others and thereby furthers the progression of disease over time.8
[Vicious vortex figure shown refers to Giam et al. 2021 .]
Neutrophilic inflammation plays an important role in the development and progression of bronchiectasis.11
Neutrophils are immune cells with antimicrobial properties.12
As neutrophils mature in the bone marrow, neutrophil serine proteases (NSPs), are activated by dipeptidyl peptidase-1 (DPP-1) and packaged into azurophil granules.13,14
Once matured, neutrophils enter the bloodstream where, upon stimuli, such as an infection, they are transported along a chemotactic gradient and exit the bloodstream at the site of infection.12,14,15
Neutrophils exert several host defence mechanisms, including antimicrobial functions such as the release of cytokines to recruit other immune cells, engulfment of microbes via phagocytosis, degranulation to release antimicrobial NSP molecules like neutrophil elastase and formation of neutrophil extracellular traps (NETs).12,14,15
Once neutrophils have executed their antimicrobial processes, they are eliminated via apoptosis to avoid further damage.14,16
However, in bronchiectasis, chronic infection and changes in the airway environment can lead to neutrophil dysregulation.17
This results in amplified numbers of neutrophils that, not only survive longer, but also lead to increased NSP activity and NET formation.10,15
NE activity and NETs have been associated with bronchiectasis disease severity and progression.18,19
This unresolved inflammatory process contributes to tissue damage, impaired mucociliary clearance, impaired bacterial phagocytosis and killing resulting in airway damage.8,16,20
Targeting the underlying neutrophilic inflammation associated with bronchiectasis continues to be an unmet need, and a comprehensive treatment approach is desirable.8,17,21
Current treatment efforts are targeted towards other components of the disease, such as antibiotics to treat chronic bacterial infection and long-term mucoactive therapies and airway clearance techniques to improve mucociliary clearance and reduce airway damage.21
However, individual treatments may not halt disease progression, as chronic neutrophilic inflammation and airway destruction can still be sustained by other stimuli.2,8
Therefore, a comprehensive multimodal treatment approach that addresses all four interrelated components of bronchiectasis may help to reduce the frequency of exacerbations in bronchiectasis patients and improve their quality of life.2,8
References
1. Chalmers JD, Chang AB, Chotirmall SH, Dhar R, McShane PJ. Bronchiectasis. Nat Rev Dis Primers. 2018;15;4(1):45. 2. Amati F, Simonetta E, Gramegna A, et al. The biology of pulmonary exacerbations in bronchiectasis. Eur Respir Rev. 2019;28(154):190055. 3. Aksamit TR, O’Donnell AE, Barker A, et al. Bronchiectasis Research Registry Consortium. Adult patients with bronchiectasis: A first look at the US bronchiectasis research registry. Chest. 2017;151(5):982–992. 4. Aliberti S, Lonni S, Dore S, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J. 2016;47(4):1113–1122. 5. Cohen R, Shteinberg M. Diagnosis and evaluation of bronchiectasis. Clin Chest Med. 2022;43(1):7–22. 6. Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the “Frequent Exacerbator Phenotype” in bronchiectasis. Am J Respir Crit Care Med. 2018;197(11):1410–1420. 7. Hill AT, Haworth CS, Aliberti S, et al. EMBARC/BRR definitions working group. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J. 2017;49(6):1700051. 8. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392(10150):880–890. 9. Metersky ML, Barker AF. The pathogenesis of bronchiectasis. Clin Chest Med. 2022;43(1):35–46. 10. Giam YH, Shoemark A, Chalmers JD. Neutrophil dysfunction in bronchiectasis: an emerging role for immunometabolism. Eur Respir J. 2021;58(2):2003157. 11. Dente FL, Bilotta M, Bartoli ML, et al. Neutrophilic bronchial inflammation correlates with clinical and functional findings in patients with noncystic fibrosis bronchiectasis. Mediators Inflamm. 2015;2015:642503. 12. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The Neutrophil. Immunity. 2021;54(7):1377–1391. 13. Cowland, J.B. and Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273(1):11–28. 14. Bekkering S, Torensma R. Another look at the life of a neutrophil. World J Hematol. 2013;2(2):44–58. 15. Keir HR, Chalmers JD. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur Respir Rev. 2022;31(163):210241. 16. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. 17. Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med. 2018;6(9):715–726. 18. Keir HR, Shoemark A, Dicker AJ, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med. 2021;9(8):873–884. 19. Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med. 2017;195(10):1384–1393. 20. Bedi P, Davidson DJ, McHugh BJ, Rossi AG, Hill AT. Blood neutrophils are reprogrammed in bronchiectasis. Am J Respir Crit Care Med. 2018;198(7):880– 890. 21. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629.
Register to receive updates and information about bronchiectasis.
Patients can use this tool to help keep track of BE symptoms and possible BE exacerbations between appointments.
Patients can use these important questions about BE as a guide to support their next doctor’s appointment.
"*" indicates required fields

7th World Bronchiectasis and NTM Conference
The 7th World Bronchiectasis and NTM Conference provides education around epidemiology, pathogenesis, diagnosis, management and treatment of bronchiectasis and nontuberculous mycobacteria (NTM).
4–6 July 2024
Dundee, Scotland

European Respiratory Society Congress 2024
The European Respiratory Society Congress is an annual event that brings together the world’s respiratory experts to showcase all the latest advances in respiratory medicine and science.
Visit Insmed at booth C7.03 Hall B
7–11 September, 2024
Vienna, Austria

This site is intended for healthcare professionals outside the US and the UK only.
© 2024 Insmed Incorporated. All Rights Reserved. Insmed, the Insmed logo and Rethink Bronchiectasis are trademarks of Insmed. All other trademarks are property of their respective owner.
Developed and funded by Insmed. NP-BE-EU-00017 06 / 2024
The site you are linking to is not controlled or endorsed by Insmed, and we are not responsible for the content. In some cases, Insmed has provided funding to support the creation of content on these websites.
Thank you for visiting Rethink Bronchiectasis.